1 Understanding Science Quiz
If you are familiar with some of these key ideas before you start SM123 it will really help you. Try the following self-assessment questions to find out how much you already know.
Advice before you start
Some of the topics in this quiz may be unfamiliar to you – if so, don’t worry. The whole point of this quiz is for you to have a go at answering each question and trying to solve them based on your current knowledge. It’s also trying to tease out your ability to work with information presented in different ways (text, figures, tables and equations etc.) so that you can assess for yourself how ready you are to start on SM123. There is some information in Part 7 to help you make that assessment. Don’t forget that if you have any concerns you can speak with one of our friendly advisers (details are given at the end of Part 7). If you are an existing student you can speak with an adviser from your Student Support Team (details can be found on your StudentHome).
a.
milli, micro, nano, giga, mega, kilo
b.
giga, mega, kilo, milli, micro, nano
c.
nano, micro, milli, kilo, mega, giga
d.
nano, micro, milli, giga, mega, kilo
The correct answer is c.
c.
Nano means a billionth; micro a millionth; milli a thousandth; kilo one thousand; mega one million and giga one billion
a.
£/kg
b.
kg/£
c.
£
d.
kg
The correct answer is a.
a.
The units for the price per unit mass are obtained by dividing the units for the money (£) by the units for the mass (kg) so the units are £ per kg or £/kg.
Question 3
a.
There is not enough space between the atoms making up the surface of the Earth.
b.
The surface of the Earth exerts a force on you that is greater than the force you exert on the Earth.
c.
The surface of the Earth exerts a force on you that is equal to the force you exert on the Earth.
The correct answer is c.
c.
The surface of the Earth exerts a force on you that is equal to the force you exert on the Earth. If the Earth exerted a force greater than the one you exert on the Earth you would be pushed upwards.
a.
hydrogen absorbs all wavelengths of white light
b.
hydrogen does not absorb any wavelengths of the white light
c.
hydrogen absorbs green and yellow light
d.
hydrogen absorbs red and blue light.
The correct answer is d.
d.
The black lines reveal the wavelengths that are missing when the light travels through hydrogen, so hydrogen must have absorbed them. These lines are in the red, blue and violet regions of the spectrum.
Question 6
Using only the following information, how many electrons are there in a sodium atom?
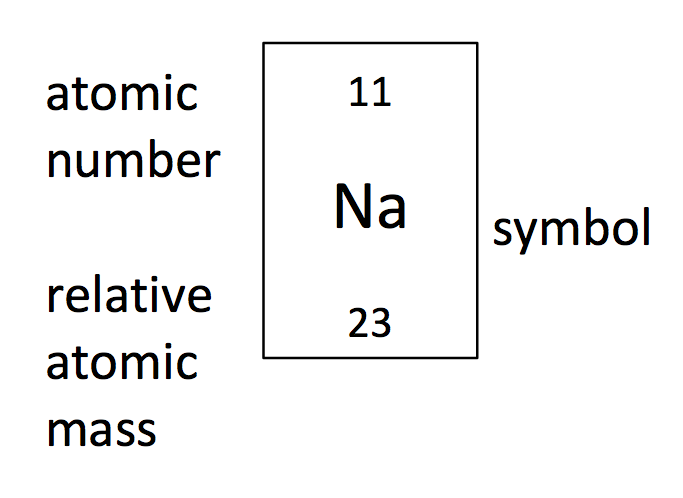
Answer
The atomic number is the number of protons in the nucleus of an atom. Atoms are neutrally charged, so the number of negatively charged electrons is equal to the number of positively charged protons. Sodium has an atomic number of 11 so the number of protons and therefore electrons in a sodium atom is 11.
a.
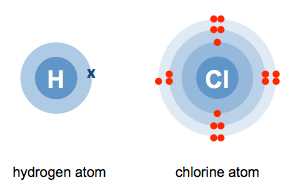
b.
H+ Cl−
c.
H–Cl
The correct answer is c.