Book 7 - From Quarks to Quasars - is going exceedingly well. I was not quite sure what to expect from a crash course in quantum physics, as it's a subject I've long been fascinated by. However, with great fascination comes great bafflement, so I was expecting to be totally befuddled.
As the great Niels Bohr said: "If quantum physics hasn't profoundly shocked you, you haven't understood it yet."
It is a source of some pleasure that in fact, I'm not struggling with this book at all. It's heavy going, and there are some difficult concepts - not to mention some downright weird concepts - but with really thorough reading it is all making sense.
Why do some concepts come so easily, while others seem so difficult? Some people struggle with ideas that others find simple; for me, that is engineering and abstract concepts. Quantum physics, though, makes perfect sense to me. I don't know what that says about the way my brain and mind work...
According to the Book, most people find it difficult to come to terms with the idea of quantum indeterminacy, with Albert Einstein himself finding the idea abhorrent.
I can understand that: Newton's world was ordered and completely predictable. I like order; I thrive on it, in fact. Some of my favourite things are lists. But quantum uncertainty makes perfect sense to me.
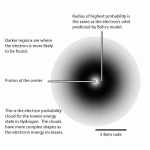
One cannot say for certain exactly where an electron is in an atom and simultaneously know its velocity, so instead of the "classic" atomic model with electrons orbiting the nucleus, Schrödinger (he of the cat - and more on him later) proposed a new and improved model. The nucleus is surrounded by a fuzzy cloud of electrons, denoting the range of possible positions the electrons may occupy.
So, why can one not predict the position and velocity of an electron? Theoretically, an experiment could be devised to measure its position or velocity, but can they be measured simultaneously?
The Heisenberg uncertainty principle says "no". If you know the exact position of a particle, you cannot know anything about its velocity at the same time. The reason why is actually rather simple, and makes perfect sense (once you understand how energy levels work in atoms...)
To measure something, you have to "see" it. And to "see" it, you have to shine a light on it. But by shining a light on an atom (which is where the electron we're looking for is) one of two things will happen
- A photon from the light may be absorbed, causing the atom to change energy levels. The quantum state of the atom will have changed, and the electron will no longer have the same position or velocity.
- The photon may not interfere with the atom at all. In which case nothing has been measured.
This is the basis from which comes the adage that the act of observation changes the observed. This is certainly true on a quantum level, and true of a subject that knows it's being watched.
(It's a good example of how armchair psychologists and philosophers grab an idea from quantum physics and give it a little tickle, without fully digesting the concept!)
The above aside brings me neatly back to Schrödinger and his beknighted cat. It's a wee niggle of mine that much of the populace seize on Schrödinger's thought experiment and bend it to a vague philosophical notion along the same line as a falling tree making a sound when there's nobody there to hear it. Or not, as the case may be.
It's not as simple as not knowing for sure if the cat in the box is dead or alive before you open the lid. (And if they're my cats, there's no uncertainty at all, because they'd be screeching threats through the lid.)
The cat in the box is awaiting its fate - a possible death by poison. A vial of poison is positioned in the box, along with a radioactive isotope. If the isotope decays, the poison will be released by a trigger system. However, here is where quantum uncertainty comes in: the isotope may decay immediately. It may not decay at all. And all the possibilities in between. But the point is that until the box is opened, nobody knows whether or not the isotope has decayed, and so whether or not the cat is dead or alive.
The isotope now has a wavefunction describing two states: decayed and undecayed (because we can predict the probability of its state at any time, but not its actual state).
Is this all making sense?
(Schrödinger had also had enough of quantum weirdness, which inspired him to anger animal rights activists the world over in the first place.)
He then carried the theory of quantum uncertainty a little further: the cat is also made of atoms, and is a quantum system (a huge and complicated one, but a system nonetheless). So, stretching this, the cat must then also have a wavefunction describing a live cat and a dead cat, using probabilities based upon the probability of the isotope's decay - because the cat's fate was now bound inextricably with that of the isotope.
Clearly, this is silly. And Schrödinger knew it, which is why he challenged the upstarts Bohr and Heisenberg and their quantum theories of oddness.
It is resolved though: the cat is shut in a box. We can have no knowledge at all of the real state of the cat: probability is just a string of numbers. Without measuring reality, we cannot describe it - so we do not try. When it comes time to open the box, we can use the probability of the isotope's decay to predict the probability of the cat's state, but beyond that - nothing.
There's an excellent book: "Quantum - A Guide for the Perplexed" by Jim Al-Khalili. It explains a lot of difficult concepts really well.
I hope I've made a start. It makes perfect sense to me, anyway!
Stay tuned for nuclear decay...