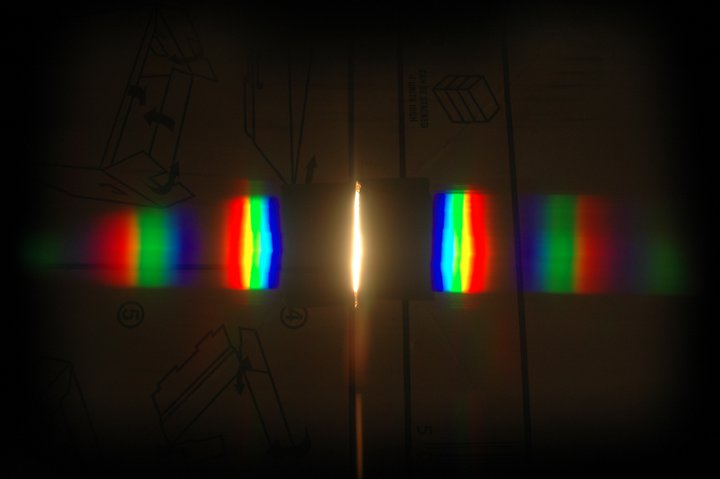
As this blog is following and documenting my adventures in science, it seems that I should say a few words about S104 Exploring Science for any prospective students of the Open University.
I'll start by stating, in no uncertain terms, that this is a Very Difficult Course, particularly if there is no (recent) background in studying science or maths. This is not a light, adult-learning-style, interest-only course: it's full on, in depth and requires an awful lot of hard work.
If any prospective students are not truly interested in science and really committed to learning, it will be extraordinarily difficult. I work full time, and I try to have a social life too - I have struggled to find the hours required for this course.
However, and I can't emphasise this enough, S104 Exploring Science is absolutely bloody brilliant. It is Professor-Brian-Cox-jazz-hands-brilliant. Finding the time to study has not been, in any way, a chore.
Some aspects of the syllabus have been easier than others; some have interested me more than others. But overall, it's fantastic.
Here, I need to pay tribute to my wonderful husband - I could not have done this without him. He has been supportive, interested, helpful (especially with the maths and physics) and he has become a very good cook. Joe's patience is seemingly never ending, and I know he's really proud of me. I am proud of him. And I am so grateful.
Anyway. Enough mush. Here are the facts, figures and ravings of an S104 Survivor.
For those thinking of starting S104, I would recommend that you do some reading first - partly to see if you really are that interested in science, and partly because it will give you a good base to build upon. I found Ben Goldacre's Bad Science to be a great introduction to scientific method, and it's a good read to boot. His blog is fab.
We Need to Talk About Kelvin by Marcus Chown is also a good read. One of the more wonderful moments of this course was when I realised, in a bolt of inspiration, that I actually understood what I had been reading about a few months earlier.
And as preparation for when you arrive, breathless and exhausted, at the bottom of the mountain that is Quantum Physics, give Jim Al-Khalili's Quantum: A Guide for the Perplexed a whirl.
In fact, just read everything you can get your hands on, in the daily media, online and in journals such as New Scientist.
Before beginning, brush up your maths. Maths used to terrify me. It's well worth doing the Open University's freebie maths book to start.
Exploring Science is a nine-month course, and the course team recommends that a minimum of 16 hours per week is put aside for study. I have found this to be fairly accurate, albeit the study time is probably an average. Most people will find some topics require far less work, while others require much more (biology and quantum physics, please stand up!) .
There are eight books covering different topics, and although the order may seem slightly odd when you first see it - it does all fall into place:
- Book 1 - Global Warming. This is a fairly gentle introduction to S104, and jumps feet-first into a subject that is bang up-to-date - climate change and all that goes with it.
- Book 2 - Earth and Space. Geology and geological processes are introduced in part one, while in part two we leave Earth and venture out into the Solar System. Again, this is not too taxing, and is a decent way to ease you in.
- Book 3 - Energy and Light. Physics-lite - I began this book reminiscing about GCSE physics, and remembering a surprising amount. By the end of the book I realised that this was Grown Up Stuff, leading my thoughts in directions they would never previously have contemplated. The maths began to pick up pace; and rather than becoming baffled and afraid, I developed a deep and abiding love for a beautiful and elegant discipline.
- Book 4 - The Right Chemistry. Again, it begins with a recap of GCSE chemistry, then steamrollered into the kind of stuff that makes you wonder if, by the end of the course, you'll be able to run your own meth lab. Fascinating. And if, like me, you were once afraid of the mole, this book will cure your fear.
- Book 5 - Life. Biology. It's the thickest book of the lot, and it's stroppy with it. Life lulls you into a false sense of security, starting with the difference between autotrophs and heterotrophs, looking at prokaryotes and eukaryotes, before steaming into the minute detail of the reactions that make up photosynthesis. Think you know how plants make their food? Think again!
- Book 6 - Exploring Earth's History. An interest in fossils and geology will mean you sail through this book. It's absolutely fascinating, and is a grand illustration of how absolutely everything in our Universe is connected. Our planet is a staggeringly beautiful and complicated place, and I am humble before it.
- Book 7 - Quarks to Quasars. Mind-bending stuff. But give it time, read everything VERY carefully, more than once, and it WILL make sense. I promise. I found that writing notes in my own words was really helpful.
- Book 8 - Life in the Universe. I'm not there yet. But the book promises to pull together all the aspects of S104, enabling us to build a complete picture of how the separate disciplines tie together. All branches of science are connected, and feed into each other. It will be good preparation for the End of Module Assessment.
Everybody's techniques for studying are different, but this is how I approached Exploring Science. As I read through each chapter, I highlighted relevant concepts, ideas and facts, making notes in my own words. I also, as you have probably gathered, began this blog. It is, in part, a method of finding out if I've fully understood what I'm learning: if others understand my tales and explanations, it's a good bet that I have.
Talk to your loved ones: bore them silly! I am lucky to have a husband who is almost as fascinated by this stuff as I am, and many of my friends are crazy about science. (I thank you all so much for listening, reading and generally being interested. I love you guys!)
Use the tools the Open University gives you: do all the activities, because they really do consolidate your learning, as well as being good fun in many cases. The questions dotted throughout the text are brilliant, testing your knowledge and understanding before you come to do the assessments.
And speaking of assessments: at the end of each book, you are required to complete an iCMA (interactive computer-marked assignment) and a TMA (tutor-marked assignment). These contribute to your overall mark, as well as helping to pull together everything you've learned.
A good tactic for the iCMAs is to write them out in rough before you enter the answers. My first one was pretty shameful, purely because I hadn't read the instructions properly! In my excitement at starting the course, I achieved only 80%...
For the TMAs - read the questions really, really carefully! Sometimes the OU examiners do not use language in the way you may expect... I found that leaving the TMAs right until the end of the book meant that I was a little stressed about getting them in on time. The questions helpfully tell you which chapters you should have finished before attempting to answer - I would advise that the TMA is completed as you go along.
Use your tutors, that's what they're there for. They are a great source of support, if you're lucky enough to get a really good, dedicated person. The tutorials are also a good source of support, as well as helping you meet other students.
Use other students too: the tutor forums can be helpful, if you get a good group - or join the S104 group on Facebook. I've made some lasting online friends through that, and it's made me laugh until I cry on more than one occasion. You are not struggling alone.
And finally: enjoy it! It's been a fantastic experience, and I'm genuinely sad at the thought of the course ending (although I cry at the news, so don't let that be a measure of normality...). Good luck, and remember:
"What we have learned is like a handful of earth. What we have yet to learn is like the whole world." Avvaiyar, Indian poet-saint.